
is an innovative medical device company that focuses on developing unique clinical solutions for coronary and peripheral vascular procedures. The initial customer response we have received to our international launch of the GuideLiner makes us very optimistic concerning the potential of this completely unique and proprietary product." Howard Root, Chief Executive Officer of Vascular Solutions, commented: "The GuideLiner catheter is a significant new internally-developed addition to Vascular Solutions' line of specialty catheters designed to meet specific needs of physicians performing percutaneous coronary interventions. Deep intubation with the GuideLiner facilitated delivery, allowed us to optimize stent apposition and also improved visualization of the vessel due to selective cannulation." We then passed a 6F GuideLiner into the vessel and by removing the proximal resistance we could then deliver the 3.0 mm stent, followed by a 4.0 mm stent and post-dilatation with a 4.0 mm balloon. Colm Hanratty, an interventional cardiologist at Belfast City Hospital in Belfast, Ireland, commented on one of his initial clinical uses of the GuideLiner: "In this patient, despite modification of the diseased segment and subsequent pre-dilatation, we could not track a 3.0 mm stent across the lesion due to significant friction in the proximal vessel. The GuideLiner is as easy to insert as a standard rapid exchange balloon catheter and has quickly become a routine part of my angioplasty practice."ĭr. Furthermore, the soft and very flexible tip will often cross tortuous disease where a stent gets stuck, enabling delivery of stents and other equipment directly to the target lesion. Douglas Fraser, an Interventional cardiologist with Manchester Heart Centre in Manchester, United Kingdom, commented on his initial clinical experience with the GuideLiner: "Deep intubation of the GuideLiner catheter within a soft 6F guide provides better backup support and is less traumatic than using stiff 7F and 8F guides that were previously required in complex disease.
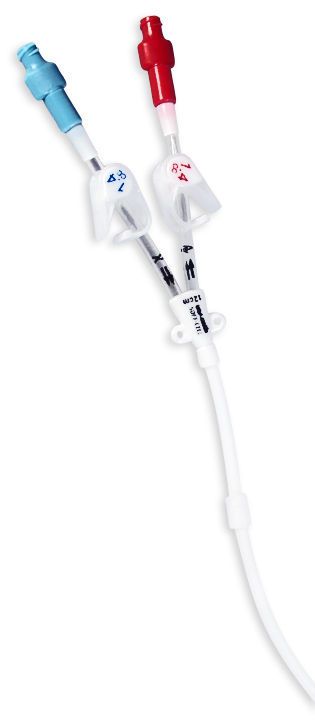
launch of the GuideLiner catheter expected to commence in November through Vascular Solutions' direct sales force.ĭr. CE mark clearance of the GuideLiner was received and European sales and clinical uses commenced in October, with the U.S. The GuideLiner catheter will be available in 6, 7 and 8 French sizes as part of Vascular Solutions' specialty catheter product line. The GuideLiner is a unique coaxial "mother and child" guide extension with rapid exchange convenience that provides back-up support and selective deep intubation in challenging coronary interventions. Food & Drug Administration to launch the GuideLiner(TM) catheter in the United States.
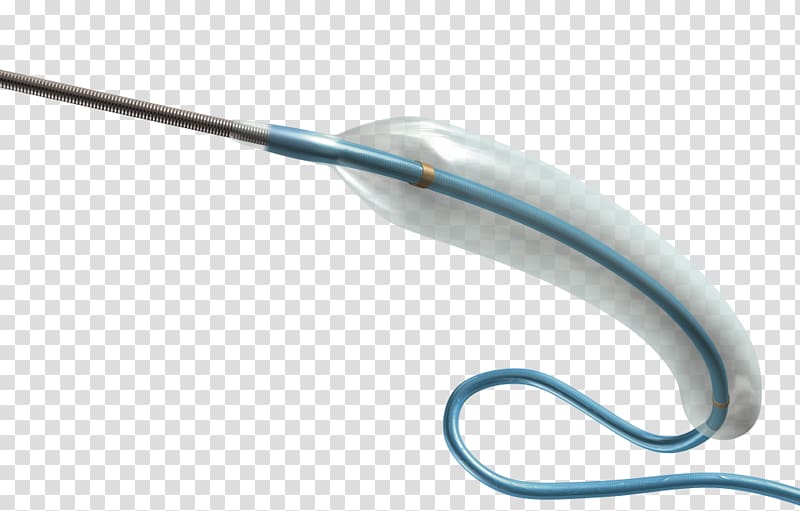
(Nasdaq:VASC) today announced that it has received 510(k) clearance from the U.S. 9, 2009 (GLOBE NEWSWIRE) - Vascular Solutions, Inc.


 0 kommentar(er)
0 kommentar(er)
